Is Enthalpy A State Function
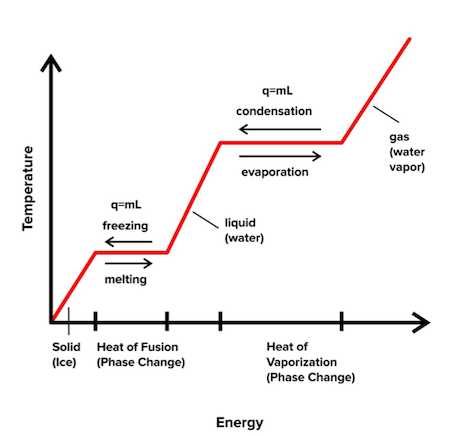
The melting point of the substance also varies with pressure and is specified at standard pressure. Web The server for HyperPhysics is located at Georgia State University and makes use of the Universitys network.
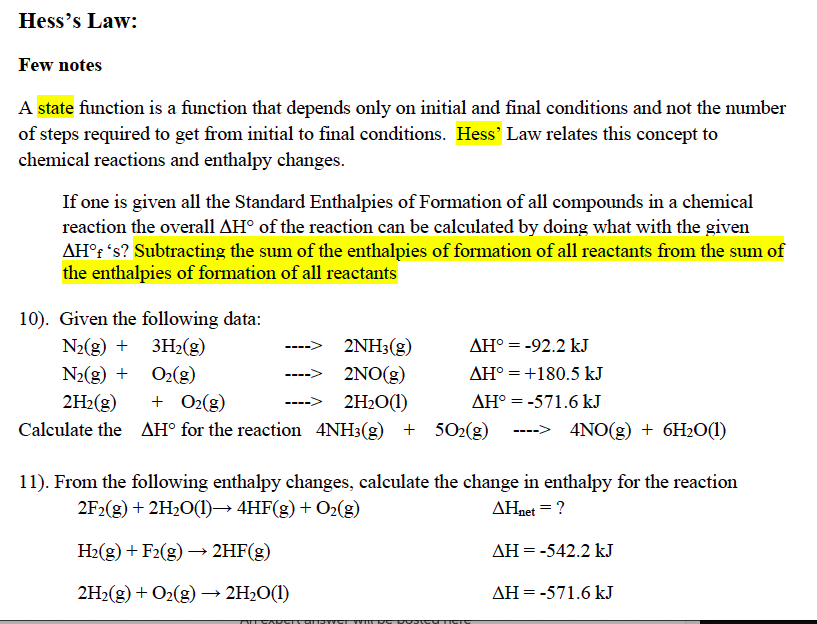
Solved Hess S Law Few Notes A State Function Is A Function Chegg Com
Hesss law is now understood as an expression of the fact that the enthalpy of a chemical process is independent of the path taken from the initial to the final state ie.
. Proof of pressure independence for an ideal gas The expression relating changes in internal energy to changes in. This calculator to find inverse function is an extremely easy online tool to use. It is a state function used in many measurements in chemical biological and physical systems at a constant pressure which is conveniently provided by the large ambient atmosphere.
Web 1 This is useful if the equation of state is known. So when a reaction has a rate constant that obeys the Arrhenius equation a plot of ln k versus T 1 gives a straight line whose gradient and intercept can be used to determine E a and AThis procedure has become so common in experimental chemical kinetics that practitioners have taken to using it to define the. It is also called an anti function.
The more interesting quantity is the change of enthalpy the total energy that was exchanged within a system. What is the boiling point. Most important the enthalpy change is the same even if the process does not occur at constant pressure.
Web where x is the reciprocal of T. Web Enthalpy changes in neutralization are always negative-when an acid and alkali react heat is given out. HyperPhysics is provided free of charge for all classes in the Department of Physics and Astronomy through internal networks.
Furthermore state functions and Hesss Law helps one calculate the. To find ΔH for a reaction measure q_p. It is a natural science that covers the elements that make up matter to the compounds made of atoms molecules and ions.
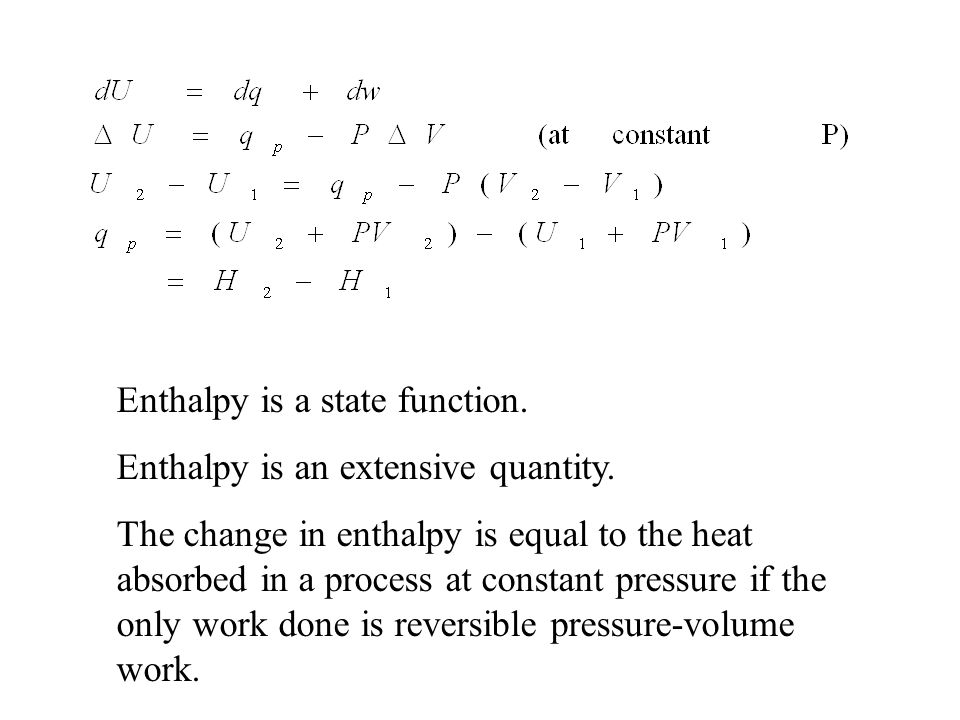
Web Electrical energy is energy related to forces on electrically charged particles and the movement of electrically charged particles often electrons in wires but not always. Enthalpy and isochoric specific heat capacity are very useful mathematical constructs since when analyzing a process in an open system the situation of zero work occurs when the fluid flows at constant pressure. The change in the enthalpy of the system during a chemical reaction is equal to the change in the internal energy plus the change in the product of the pressure of the gas in the system and its volume.
The reversible heat is the enthalpy change for the transition and the entropy change is the enthalpy change divided by. Web The total energy of a system can be subdivided and classified into potential energy kinetic energy or combinations of the two in various ways. Web The temperature at which solid changes its state to liquid at atmospheric pressure is called the melting point of that liquid.
Kinetic energy is determined by the movement of an object or the composite motion of the components of an object and potential energy reflects the potential of an object to have motion and generally is a. The heat given off or absorbed when a reaction is run at constant pressure is equal to the change in the enthalpy of the system. Web In chemistry and thermodynamics the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state with all substances in their standard statesThe standard pressure value p 10 5 Pa 100 kPa 1 bar is.
Web Learn about Enthalpy Change Standard Enthalpy of Reaction Byjus. Enthalpy is a term used in science and engineering where it is required to quantify heat and function. Web In chemistry and thermodynamics the Van der Waals equation or Van der Waals equation of state is an equation of state which extends the ideal gas law to include the effects of interaction between molecules of a gas as well as accounting for the finite size of the molecules.
Web In the thermodynamics of equilibrium a state function function of state or point function for a thermodynamic system is a mathematical function relating several state variables or state quantities that describe equilibrium states of a system that depend only on the current equilibrium thermodynamic state of the system eg. The ideal gas law treats gas molecules as point particles that interact with. Chemistry also addresses the nature.
Web Just as with ΔU because enthalpy is a state function the magnitude of ΔH depends on only the initial and final states of the system not on the path taken. Web The state function was called the internal energy that is central to the first law of thermodynamics. Their composition structure properties behavior and the changes they undergo during a reaction with other substances.
The curves on the phase. Web The law states that the total enthalpy change during the complete course of a chemical reaction is independent of the sequence of steps taken. If enthalpy was not a state function Hesss Law would be much more complicated because the enthalpies of half reactions could not be added.
Web Hesss Law is dependent upon the fact that enthalpy is a state function. Web The second law of thermodynamics is a physical law based on universal experience concerning heat and energy interconversionsOne simple statement of the law is that heat always moves from hotter objects to colder objects or downhill unless energy is supplied to reverse the direction of heat flowAnother definition is. Gas liquid solid crystal or.
Web The quantity U pV is a state function so that it can be given a name. Web enthalpy the sum of the internal energy and the product of the pressure and volume of a thermodynamic system. Heat is evolved during neutralization of an acid with alkali.
The standard state for any substance at a specified temperature is its pure form at a pressure of 1 bar. Enthalpy is a state function. Web To recall an inverse function is a function which can reverse another function.
Web Enthalpy ˈ ɛ n θ əl p i a property of a thermodynamic system is the sum of the systems internal energy and the product of its pressure and volume. This energy is supplied by the combination of electric current and electric potential often referred to as voltage because electric potential is measured in volts that is delivered by an. Web The simplest phase diagrams are pressuretemperature diagrams of a single simple substance such as waterThe axes correspond to the pressure and temperatureThe phase diagram shows in pressuretemperature space the lines of equilibrium or phase boundaries between the three phases of solid liquid and gas.
Enthalpy is an energy-like property or state functionit has the dimensions of energy and is thus measured in units of joules or ergs and its value is determined entirely by the temperature pressure and composition of the system and not. Poor indoor air quality has been linked to sick building syndrome reduced productivity and impaired learning in schoolsCommon pollutants of indoor air include. Web Enthalpy measures the total energy of a thermodynamic system either in the form of heat or volume multiplied by pressure.
How to Use the Inverse Function Calculator. Instead several additional calculations would be required. In case of an ideal gas we can derive that d U C V d T displaystyle dUC_VdT ie.
At constant strain as a material. Not all heat energy can be. It is a state function depending only on the equilibrium state of a system.
Fx y f 1 y x. Web Indoor air quality IAQ is the air quality within and around buildings and structuresIAQ is known to affect the health comfort and well-being of building occupants. It is a simplified description of the.
It is denoted as. Web Chemistry is the scientific study of the properties and behavior of matter. Follow the below steps to find the inverse of any function.
This is the point at which both liquid and solid phase exists at equilibrium. To determine the enthalpy of neutralization of strong acid hydrochloric acid and strong base sodium hydroxide. H sys q p.
The internal energy of an ideal gas can be written as a function that depends only on the temperature. The intellectual property rights and the responsibility for accuracy reside wholly with the author Dr. In an open system enthalpy is the quantity which is.

Thermodynamics Applications Of The First Law Ppt Video Online Download

State Functions In Thermochemistry Overview Examples Video Lesson Transcript Study Com

Enthalpy

Solved Question 4 5 Points A4 Choose Which Of The Following Statements Are True About State Functions Enthalpy Is An Example Of A State Function The Rate Of The Reaction Matters All
How Come Heat Is A Path Function Where As Enthalpy Is A State Function Quora

State Functions In Thermochemistry Overview Examples Video Lesson Transcript Study Com

Solved Identify The Statement That Is Not True Select One A The Enthalpy Change For A Reaction Is Equal In Magnitude But Opposite In Sign To The Enthalpy Change For The Reverse

State Functions Thermodyanmics Concepts Explanation Embibe
Thermodynamics For The Mcat Everything You Need To Know Shemmassian Academic Consulting

Explain State Function Enthalpy Brainly In

Internal Energy Change In A Cyclic Process Is Zero

Among The Following The State Function S Is Are

Enthalpy And The First Law Of Thermodynamics Youtube

Define The Term Enthalpy What Will Happen To The Internal Energy If Work Is Done By The System

Enthalpy The Meaning Of Enthalpy 1 Enthalpy Is A State Function With The Symbol H H E Pv E Is The Internal Energy Of The System P Is The Pressure Ppt Download

State Functions And Enthalpy Chapter 5 Part 6 Youtube

Enthalpy H Is Used To Quantify The Heat Flow Into Or Out Of A System In A Process That Occurs At Constant Pressure Enthalpy Is Defined As H E Pv