10+ Chapter 5.1 Electrons In Atoms Answer Key
Web NCERT Solutions Class 9 Science Chapter 3 Atoms And Molecules prepared by expert teachers at BYJUS according to the 2022-23 syllabus of the CBSE exams. NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom at BYJUS provide students with solutions to all the questions provided in the NCERT Class 9 textbook recommended by the CBSE boardThis article provides students with study material that.

Chapter 5 Review Answers
44 Newtons Third Law of Motion.
. In this extreme case the rest mass in Equation 629 must be changed to the rest mass of the atom. Concept of a System. Web NCERT Solutions for Class 9 Science Chapter 4 CBSE Free PDF Download.
Download Free PDF View PDF. 11 It is the foundation of all quantum physics including quantum chemistry quantum field theory quantum technology and quantum information science. Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour.
Class 12 Physics NCERT Solutions for Chapter 13 Nuclei. If the ionization reaction is essentially complete the acid or base is termed strong. Thus many students find it confusing that for example the 5p orbitals fill immediately after the 4d and immediately before the 6s.
To understand how the major forms of light differ in wavelength frequency and energy. The complete range of all possible forms of light is called the electromagnetic spectrum so-named because light carries both. Web Acid and Base Ionization Constants.
This rock retains its shape because of the forces holding its atoms or molecules together. The NCERT Solutions for Class 12 Chapter 3 Current Electricity is an essential resource material that is needed by all students to score good marks in Class 12 Physics CBSE examinations and entrance examinations. Web Microsoft pleaded for its deal on the day of the Phase 2 decision last month but now the gloves are well and truly off.
Microsoft describes the CMAs concerns as misplaced and says that. The atoms lose their electrons more easily. Web Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more.
The mass of uranium needed per year is 3076 x 10 4 Kg. Web These observations are consistent with i the oxidation of elemental copper to yield copperII ions Cu 2 aq which impart a blue color to the solution and ii the reduction of silverI ions to yield elemental silver which deposits as a fluffy solid on the copper wire surfaceAnd so the direct transfer of electrons from the copper wire to the aqueous. Modification of work by the Italian voiceFlickr.
Download Free PDF View PDF. If relatively little ionization occurs the acid or base is weakAs will be evident throughout the remainder of this chapter there are many more weak. Web Electrical resistivity also called specific electrical resistance or volume resistivity is a fundamental property of a material that measures how strongly it resists electric currentA low resistivity indicates a material that readily allows electric current.
The correct answer is c. 232 Digestive System Processes and Regulation. 076 x 10 4 Kg.
233 The Mouth Pharynx and Esophagus. For larger atoms the most loosely bound electron is located farther from the. 237 Chemical Digestion and Absorption.
236 Accessory Organs in Digestion. 42 Newtons First Law of Motion. 231 Overview of the Digestive System.
Web For example calcium is a group 2 element whose neutral atoms have 20 electrons and a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. The Electromagnetic Spectrum Learning Goal. 45 Normal Tension and Other Examples of Force.
234 The Stomach. Download Free PDF View PDF. The atoms lose their electrons more easily is a wrong statement because as we move from left to right across the periods of the periodic table the non-metallic character increases.
Modification of work by vxlaFlickr. For a positive ion such as NO we add the number of valence electrons on the atoms in the ion and then subtract the. Mass of 6023 x 10 23 atoms of 235 U 235 x 10-3 kg.
The relative strength of an acid or base is the extent to which it ionizes when dissolved in water. The NCERT Solutions for Class 12 Physics PDF provided here. A Closer Look.
Classical physics the collection. Web c The atoms lose their electrons more easily. Web Connection for AP Courses.
Mass of 788400 x 10 23 atoms of 235 U 235 x 10-36023 x 10 23 x 78840 x 10 24 3. Click here to get Class 9 Science NCERT Solutions Chapter 3 Atoms And Molecules. 43 Newtons Second Law of Motion.
Download Free PDF View PDF. The Liver Pancreas and Gallbladder. 235 The Small and Large Intestines.
Web 1 mole ie 235 g of 235 U contains 6023 x 10 23 atoms. Web Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. This type of.
When a Ca atom loses both of its valence electrons the result is a cation with 18 electrons a 2 charge and an electron configuration of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6. Web Figure 118 a Particles in a solid always have the same neighbors held close by forces represented here by springs. Web CHAPTER 15 ACIDS AND BASES.
These particles are essentially in contact with one another. D The oxides become more acidic. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.
Web Study with Quizlet and memorize flashcards containing terms like Ranking Task. 47 Further Applications of Newtons Laws of. Energy is always required to remove electrons from atoms or ions so ionization processes are endothermic and IE values are always positive.
Web NCERT Solutions for Class 12 Physics Chapter 3 Free PDF Download. The filling order is based on observed experimental results and has been confirmed by theoretical calculations. Resistivity is commonly represented by the Greek letter ρ The SI unit of electrical resistivity is the ohm-meter.
Web Water is the chemical substance with chemical formula H 2 O. Photons that collide with the inner electrons of the target atoms in fact collide with the entire atom. If the atoms that form a covalent bond are identical as in H 2 Cl 2 and other diatomic molecules then the electrons in the bond must be shared equallyWe refer to this as a pure covalent bondElectrons shared in pure covalent bonds have an equal probability of being near each nucleus.
Web Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Web These interactions can be expressed as couplings to some macroscopic variables of the object described by quantum waves well localized in 3D-space which are in a product with the relative variables state of the object like entangled electrons in atoms and other parts of the object see Vaidman 2019 Sec. Please contact Savvas Learning Company for product support.
A rock is an example of a solid. Web The nonshifted peak shown in Figure 612 is due to photon collisions with tightly bound inner electrons in the target material. 41 Development of Force Concept.

Putting Anion P Interactions Into Perspective Frontera 2011 Angewandte Chemie International Edition Wiley Online Library

Andrew Neil It S Madness To Ignore The Answer To The Energy Crisis That S Lying Under Our Feet Not A Lot Of People Know That
Download Vol 5 As Pdf Facility For Antiproton And Ion Research

Chapter 5 Electrons In Atoms 5 1 Revising The Atomic Model Ppt Download
High Resolution Momentum Imaging From Stern S Molecular Beam Method To The Coltrims Reaction Microscope Springerlink

Chem12 C0500 Ctbs Name Date Class Electrons In Atoms Chapter Test B A Matching Match Each Term In Column B With The Correct Description In Column A Course Hero

5 Harnessing Quantum Dynamics In The Time And Frequency Domains Manipulating Quantum Systems An Assessment Of Atomic Molecular And Optical Physics In The United States The National Academies Press

What Is The Maximum Number Of Electrons In An Atom That Can Have The Quantum Numbers N 4 M 1
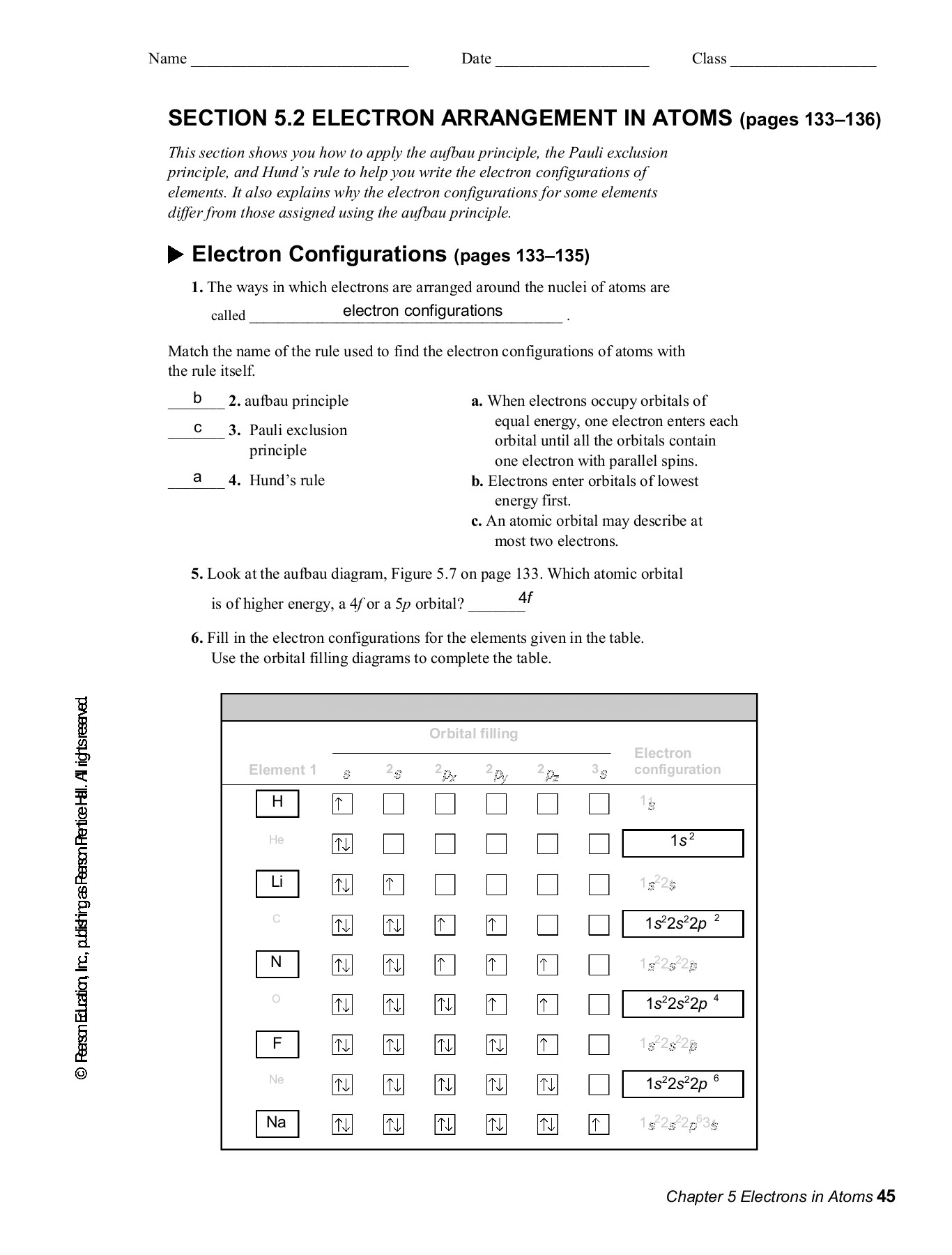
Electrons In Atoms 5 North Plainfield School District Guset User Flip Pdf Anyflip

Electron Config Key Chemistry Worksheet Configurations Answer Key Neatly Provide The Best Complete Detailed Yet Concise Answers To The Following Course Hero

Jee Main 2013 Question Paper And Solution

Electrons In Atoms 5 North Plainfield School District Guset User Flip Pdf Anyflip
If The Electron Of A Hydrogen Atom Is Making 6 26 10 15 Revolution Per Second How Much Is The Current It Constituted Quora

What Is The Energy Required To Move The Electron From The Ground State Of H Atom To The First Excited State Given That The Ground State Energy Of H Atom Is 13 6

Complete Chemistry Notes Chemistry Year 11 Hsc Thinkswap
Ncert Exemplar Class 8 Maths Solutions Chapter 8 Exponents Powers
If The Electron Of A Hydrogen Atom Is Making 6 26 10 15 Revolution Per Second How Much Is The Current It Constituted Quora